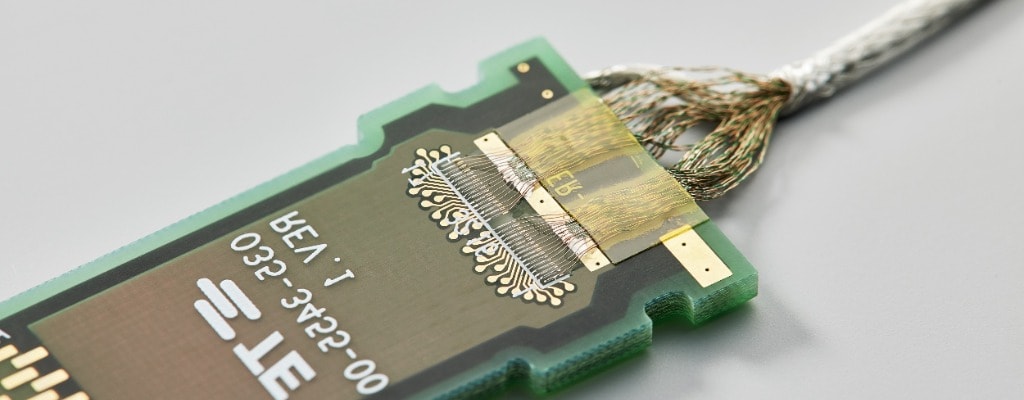
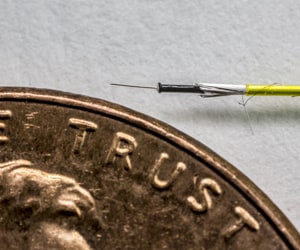
Fine Wire Automation – Device Talks Webinar
Listen to Troy Brown, Staff Manufacturing and Development Engineer, Advanced Development, discuss the applications and benefits of fine wire automation and provide an overview of our automated fine wire management processes and technical capabilities.